
In addition to fission products, waste also includes some heavy nuclei that come from non-fission neutron absorption: neptunium, americium and curium. The vast majority of fission products have no use and constitute the bulk of “high-level” radioactive waste (we will discuss their management below). Fission products are the mixture of surviving fragments and the descendants of these fragments. Most are radioactive and decay more or less quickly, giving rise to other nuclei, often also radioactive. Now let us look at the fission fragments. These nuclei, called fertile, are thorium 232Th which becomes uranium 233U, and uranium 238U which becomes plutonium 239Pu. Some other heavy nuclei are not fissile, but after absorption of a neutron they disintegrate by beta radioactivity and their resulting “grandson” is fissile. This phenomenon plays a keyrole in the operation of most nuclear reactors, those called thermal reactors, as opposed to “fast neutron reactors” ( FNR ). This slowing is called moderation or thermalisation, because the neutron velocity decreases to the level that thermal agitation confers on the nuclei of the moderator material. It was Enrico Fermi who first observed (in 1934) that if neutrons were slowed down by elastic shocks on light nuclei (paraffin) before being directed towards a target material, the latter would start to absorb much more neutrons. If the number of neutrons is kept constant, the power released is stable: this is what is achieved in a nuclear power plant. If this chain develops exponentially, it very quickly releases an enormous amount of energy: this is the principle of atomic bombs.
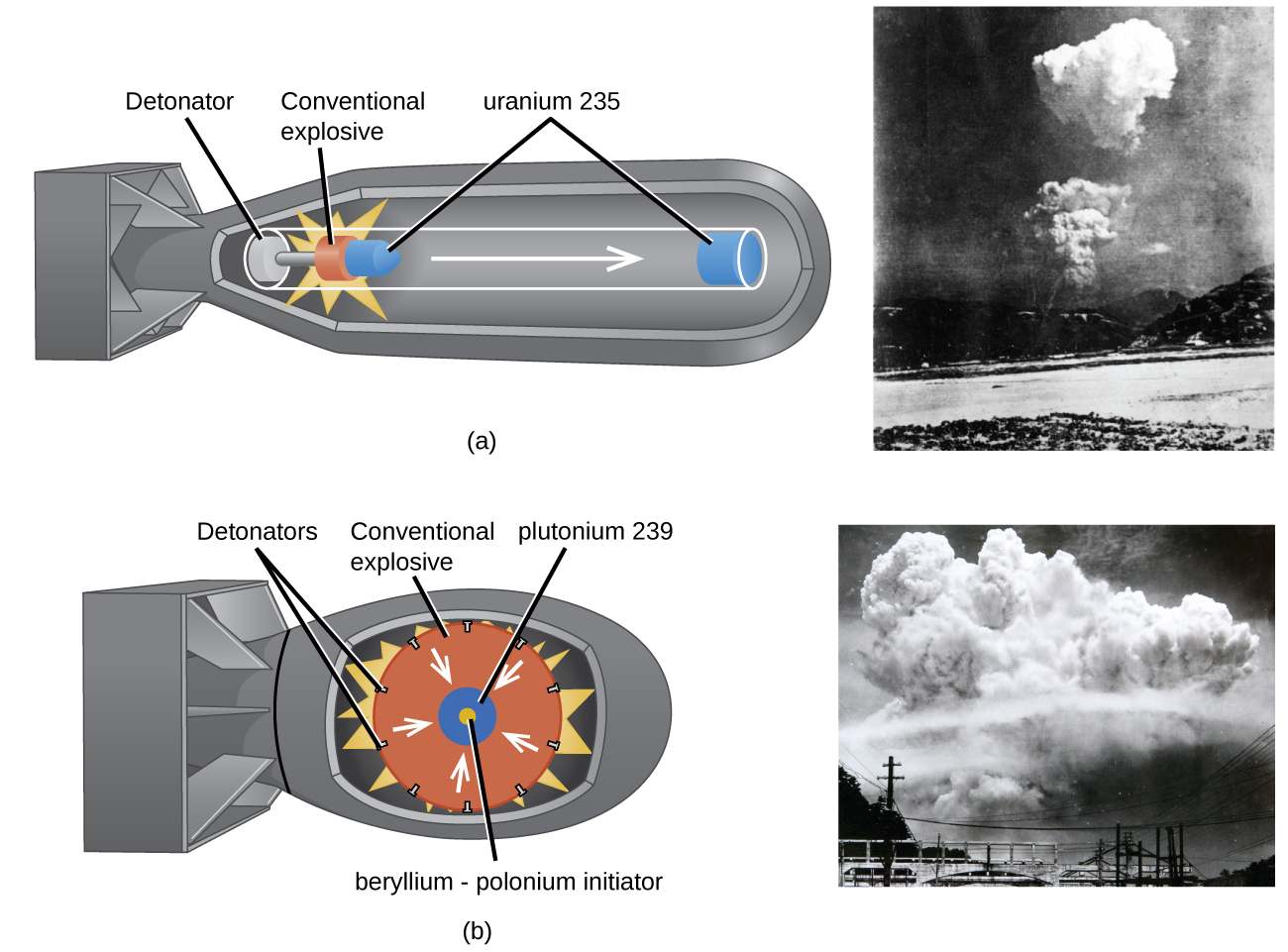
The fission of one gram of uranium thus produces more energy than the combustion of one ton of oil.Īfter fission, if the new neutrons encounter other fissile nuclei, those may in turn split, giving rise to a chain reaction. A fission releases 200 million electron volts (200 MeV). An elementary chemical reaction typically releases an energy of a few electron volts (eV). We shall see that nuclear reactors called power reactors use this heat to produce electricity. This energy appears mainly in the form of kinetic energy from fragments that separate at high speed, but when those shake up neighbouring atoms, everything is transformed into heat (see “ Energy“). The sum of the masses of the fission fragments is less than the sum of the masses of the initial nucleus and the neutron it absorbed: as Einstein taught us, this small loss of mass corresponds to a huge release of energy, according to the famous formula E = mc 2 that it would be more correct to write ∆E = -c 2∆m, where ΔE represents the released energy, Δm, the difference between the final and initial mass, and c, the speed of light in vacuum. During the fission process, a few neutrons, 2 or 3, are ejected at high speed, typically 20,000 km/s. The fission of a uranium 235 nucleusWhen a few very heavy nuclei (uranium 233U and 235U, plutonium 239Pu and 241Pu) absorb a neutron, they are so destabilized that they usually split into two fragments: this is nuclear fission. Before discussing this text, it is advisable to read the article “ Radioactivity and nuclear reactions“. In this article, we will describe the fission reaction that causes the energy used by about 450 nuclear reactors worldwide to produce 11% of the world’s electricity, the operation of these types of machines, and their positive and negative impacts on the environment. Radioactive waste is produced in limited volumes, which allows for its containment in the very long term, but its management remains controversial.

Since the 1950s, reactors have evolved into “generations”, learning from the rare accidents to enhance their safety and robustness. During normal operation, nuclear reactors are quite environmentally friendly, particularly with regard to greenhouse gases.
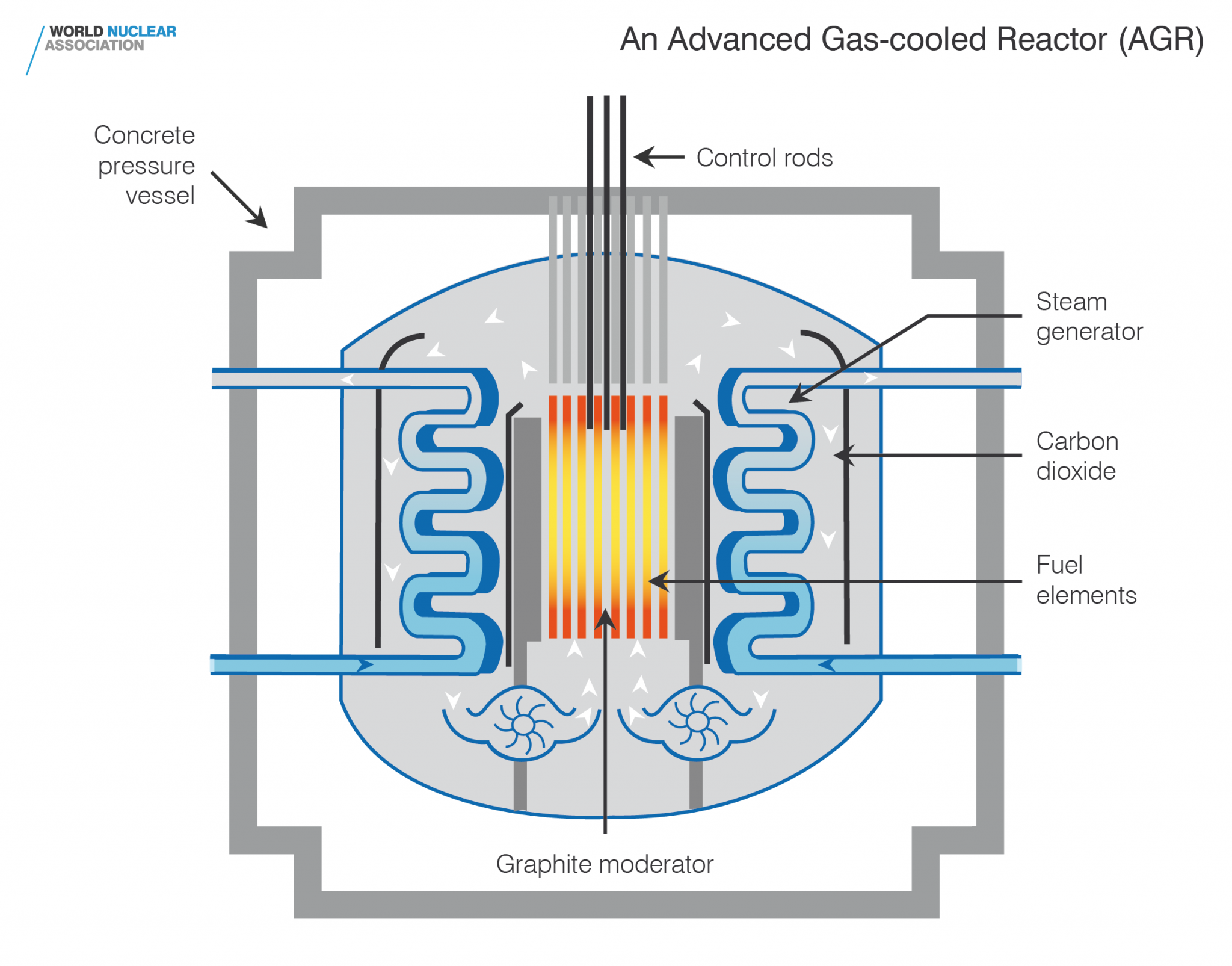
This electricity production can be adjusted according to demand. A nuclear reactor converts into electricity the heat produced by the fission of uranium nuclei.


 0 kommentar(er)
0 kommentar(er)
