
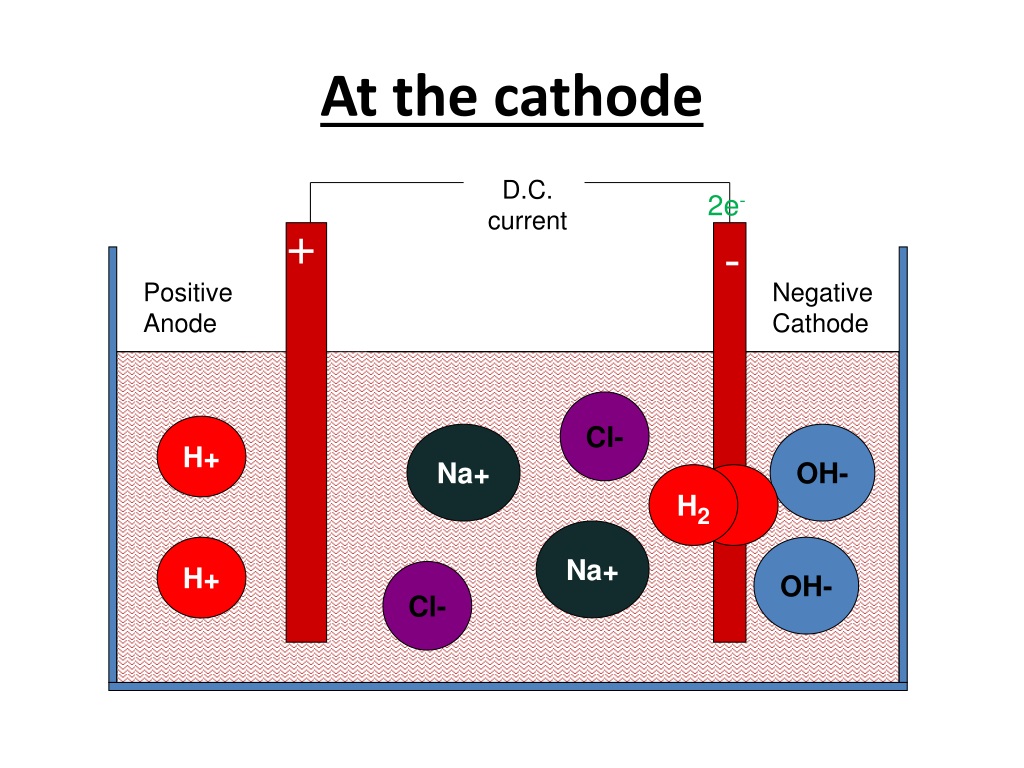
What happened at the other electrode (A) 0.05 mole of Cu2+ ions passed into solution (B) 0.112 litre of Cl, was liberated (C) 0.56 litre 0, was liberated (D) 0. Physics Wallah strives to develop a comprehensive pedagogical structure for students, where they get a state-of-the-art learning experience with study material and resources. In the electrolysis of CuCl2 solution using Cu electrodes the mass of cathode increases by 3.18g.

With our affordable courses like Lakshya, Udaan and Arjuna and many others, we have been able to provide a platform for lakhs of aspirants.įrom providing Chemistry, Maths, Physics formula to giving e-books of eminent authors like RD Sharma, RS Aggarwal and Lakhmir Singh, PW focuses on every single student's need for preparation. Physics Wallah's main focus is to make the learning experience as economical as possible for all students. But the name 'copper (II)' is a massive clue and all. We believe in empowering every single student who couldn’t dream of a good career in engineering and medical field earlier. Example 2: Copper (II) chloride solution in water (where the electrodes are inert) If you are asked about the electrolysis of this solution in an exam you would not be expected to know the details of the shell arrangement for the element copper (because we only need the first 20 elements).

PW strives to make the learning experience comprehensive and accessible for students of all sections of society. We successfully provide students with intensive courses by India's top faculties and personal mentors. What happened at the other electrode (A) 0.05 mole of Cu2+ ions passed into solution (B) 0. Physics Wallah also caters to over 3.5 million registered students and over 78 lakh+ Youtube subscribers with 4.8 rating on its app. In the electrolysis of CuCl2 solution using Cu electrodes the mass of cathode increases by 3.18g. Chloride ions lose electrons (oxidation) to form chlorine atoms. We also provide extensive NCERT solutions, sample papers, NEET, JEE Mains, BITSAT previous year papers, which makes us a one-stop solution for all resources. The overall reaction is M g C l 2 (l) M g (g) + C l 2 (g) (ii) For Calcium chloride C a 2 + 2 e C a (calcium metal at the cathode) 2 C l 2 e c l 2 (chlorine gas at the anode) Calcium ions gain electrons (reduction) to form calcium atoms. Physics Wallah is India's top online ed-tech platform that provides affordable and comprehensive learning experience to students of classes 6 to 12 and those preparing for JEE and NEET exams.


 0 kommentar(er)
0 kommentar(er)
